In-House Laboratory

Our analysis list (Vademecum) provides an overview of all tests offered in-house. Further information on the respective analyses can be found there, such as type of sample collection, storage and analysis time.
The ZRM diagnostic laboratory is authorized by the Therapeutic Products Control Authority of the Canton of Zurich and by Swissmedic.
Head of the ZRM: Prof. Dr. med. Jan Fehr
Head of the ZRM laboratories: Prof. Dr. Michael Berney
Head of the diagnostic laboratory: Dr. sc. nat. FAMH Cornelia Ottiger
ZRM Diagnostic Laboratory Vademecum
More detailed information on the tax points and prices of the respective analyses can be found in the Analysis List (AL) of the Federal Office of Public Health (FOPH).
|
Hepatitis B vaccination status (anti-HBs titer) |
|
| Method | Enzyme immunoassay fluorescence detection (ELFA) |
| Availability | Monday - Friday |
| Sampling |
VACUETTE® TUBE 8ml CAT Serum Separator Coagulation Activator
|
| Material | Serum, (exceptionally heparin plasma / EDTA plasma) |
| Preparation of patients | none |
| Volume | 0.5 ml |
| Storage temperature | 4-8°C |
| Storage duration | 5 days |
| Long-term storage | <-20°C |
| Disruptive factors | Hemolysis, lipemia, jaundice |
| Reference range | Vaccination protection: <10 IU/l none; 10-100 short-term; >100 long-term; >1000 long-term |
| Analysis time (with preparation) | approx. 80 min |
| AL position number | 3057.00 |
| Remark / Special | Determination of anti-HBs antibodies |
|
Measles antibodies (anti-measles IgG) |
|
| Method | Enzyme immunoassay fluorescence detection (ELFA) |
| Availability | Monday - Friday |
| Sampling |
VACUETTE® TUBE 8 ml CAT Serum Separator Coagulation Activator
|
| Material | Serum |
| Preparation of patients | none |
| Volume | 0.5 ml |
| Storage temperature | 4-8°C |
| Storage duration | 5 days |
| Long-term storage | <-20°C |
| Disruptive factors | Hemolysis, lipemia, jaundice |
| Reference range | IgG neg / pos |
| Analysis time (with preparation) | approx. 60 min |
| AL position number | 3122.00 |
| Remark / Special | Measles, Rubeola |
|
Mumps antibodies (anti-mumps IgG) |
|
| Method | Enzyme immunoassay fluorescence detection (ELFA) |
| Availability | Monday - Friday |
| Sampling |
VACUETTE® TUBE 8 ml CAT Serum Separator Coagulation Activator
|
| Material | Serum |
| Preparation of patients | none |
| Volume | 0.5 ml |
| Storage temperature | 4-8°C |
| Storage duration | 5 days |
| Long-term storage | <-20°C |
| Disruptive factors | Hemolysis, lipemia, jaundice |
| Reference range | IgG neg / pos |
| Analysis time (with preparation) | approx. 60 min |
| AL position number | 3128.00 |
| Remark / Special | Parotitis |
| Rubella antibody (anti-rubella IgG) | |
| Method | Enzyme immunoassay fluorescence detection (ELFA) |
| Availability | Monday - Friday |
| Sampling |
VACUETTE® TUBE 8 ml CAT Serum Separator Coagulation Activator
|
| Material | Serum |
| Preparation of patients | none |
| Volume | 0.5 ml |
| Storage temperature | 4-8°C |
| Storage duration | 7 days |
| Long-term storage | <-20°C |
| Disruptive factors | Hemolysis, lipemia, jaundice |
| Reference range | IgG neg / pos |
| Analysis time (with preparation) | approx. 60 min |
| AL position number | 3167.00 |
| Remark / Special | Rubella |
|
Varicella antibodies (anti-varicella IgG, anti-VZV IgG) |
|
| Method | Enzyme immunoassay fluorescence detection (ELFA) |
| Availability | Monday - Friday |
| Sampling |
VACUETTE® TUBE 8 ml CAT Serum Separator Coagulation Activator
|
| Material | Serum |
| Preparation of patients | none |
| Volume | 0.5 ml |
| Storage temperature | 4-8°C |
| Storage duration | 5 days |
| Long-term storage | <-20°C |
| Disruptive factors | Hemolysis, lipemia, jaundice |
| Reference range | IgG neg / pos |
| Analysis time (with preparation) | approx. 60 min |
| AL position number | 3178.00 |
| Remark / Special | Chickenpox/herpes zoster |
|
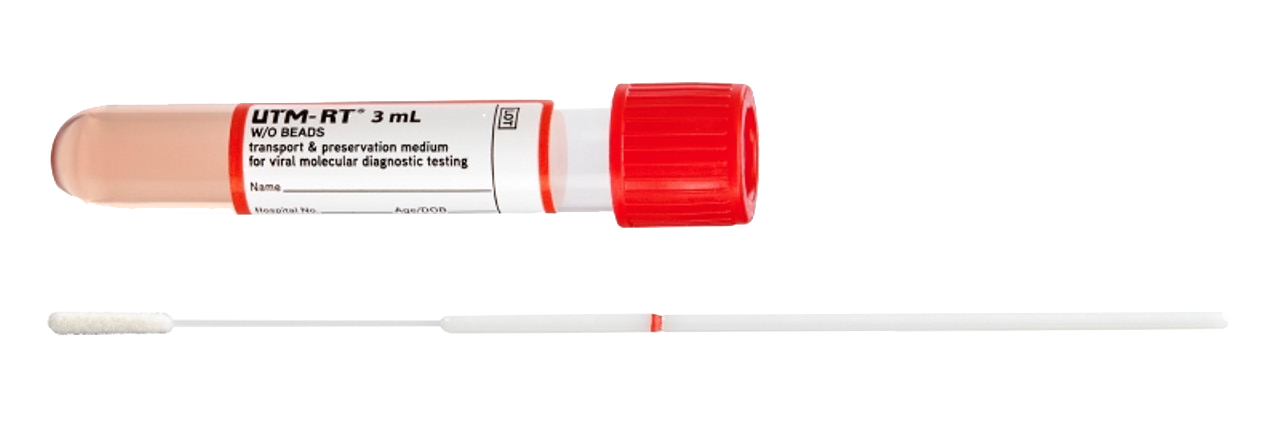
SARS-Coronavirus-2 PCR |
|
| Method | PCR |
| Availability | Monday - Friday |
| Sampling |
Copan 305VN (Nasopharyngeal swab), E-swab
|
| Material | Nasopharyngeal swab |
| Preparation of patients | none |
| Volume | 0.5 ml |
| Storage temperature | 4-8 °C |
| Storage duration | 7 days |
| Long-term storage | -80°C |
| Reference range | No pathogen detection, i.e. negative |
| Analysis time (with preparation) | approx. 40 min |
| AL position number | 3186.00 |
| Remark / Special | SARS-2/CoV19 |
| Chlamydia trachomatis (CT) PCR |
|
| Method | PCR |
| Availability | Monday - Friday |
| Sampling |
For urine samples: BD Vacutainer (Ref. 364941)
BD Vacutainer Z (Ref. 365000)
For smear tests: Copan E-Swabs
PLP tube with 1mL, liquid Amies medium (492CE03)
eSwab for pharyngeal swabs (C503CS01)
eSwab for vaginal swabs (C502CS01)
eSwab for rectal swabs (C502CS01)
eSwab for urethral swabs (C525CS01)
|
| Material | pharyngeal/vaginal/rectal/urethral swab or urine sample |
| Preparation of patients | none |
| Volume | 1 ml |
| Storage temperature | 4-8 °C |
| Storage duration | 2 days |
| Long-term storage | -80°C |
| Reference range | No pathogen detection, i.e. negative |
| Analysis time (with preparation) | ca. 100 min |
| AL position number | 3396.00 / 3460.10 |
| Remark / Special | Chlamydia trachomatis |
| Neisseria gonorrhoeae (NG) PCR | |
| Method | PCR |
| Availability | Monday - Friday |
| Sampling |
For urine samples: BD Vacutainer (Ref. 364941)
BD Vacutainer Z (Ref. 365000)
For smear tests: Copan E-Swabs
PLP tube with 1mL, liquid Amies medium (492CE03)
eSwab for pharyngeal swabs (C503CS01)
eSwab for vaginal swabs (C502CS01)
eSwab for rectal swabs (C502CS01)
eSwab for urethral swabs (C525CS01)
|
| Material | pharyngeal/vaginal/rectal/urethral swab or urine sample |
| Preparation of patients | none |
| Volume | 1 ml |
| Storage temperature | 4-8 °C |
| Storage duration | 1 day |
| Long-term storage | -80°C |
| Reference range | No pathogen detection, i.e. negative |
| Analysis time (with preparation) | ca. 100 min |
| AL position number | 3460.00 / 3396.10 |
| Remark / Special | Neisseria gonorrhoeae |
| Mycoplasma genitalium (MG) PCR (inklusive Makrolid-Resistenz) | |
| Method | PCR |
| Availability | Monday - Friday |
| Sampling |
For urine samples: BD Vacutainer (Ref. 364941)
BD Vacutainer Z (Ref. 365000)
PLP tube with 1mL, liquid Amies medium (492CE03)
For smear tests: Copan E-Swabs
eSwab for pharyngeal swabs (C503CS01)
eSwab for vaginal swabs (C502CS01)
eSwab for rectal swabs (C502CS01)
eSwab for urethral swabs (C525CS01)
|
| Material | pharyngeal/vaginal/rectal/urethral swab or urine sample |
| Preparation of patients | none |
| Volume | 1 ml |
| Storage temperature | 4-8 °C |
| Storage duration | 2 days |
| Long-term storage | -80°C |
| Reference range | No pathogen detection, i.e. negative |
| Analysis time (with preparation) | ca. 130 min |
| AL position number | 3455.00 / 3349.00 (resistance testing) |
| Remark / Special | Mycoplasma genitalium |


2-2-1.png?width=250&height=104&name=BD_364915_IMG_0615%20(2)2-2-1.png)

x.png?width=816&height=112&name=eSwab%20f%C3%BCr%20pharyngealer%20Abstrich%20(C503CS01)x.png)
x.png?width=800&height=128&name=eSwab%20f%C3%BCr%20vaginaler%20Abstrich%20(C502CS01)x.png)
x.png?width=778&height=92&name=eSwab%20f%C3%BCr%20urethraler%20Abstrich%20(C525CS01)x.png)